

PD-L1 is expressed by tumor cells and can bind with PD-1 to inhibit the activation of T cell and cytokine production. In 1992, PD-1 was first discovered in mice ( 13) as a member of the CD28 superfamily, and PD-L1 (also known as CD274 or B7-H1) was its ligand ( 14). Therefore, improving the efficacy of cervical cancer treatment has been a major medical challenge worldwide. Thus, choosing proper therapeutic strategies for patients with recurrent/metastatic cervical cancer remains challenging and is a tough issue in the treatment of gynecological tumors ( 11, 12). The effects of traditional chemotherapy are unsatisfactory, and there are various complications related to surgery and radiotherapy. In addition, the treatment approaches are quite limited. Compared with the cervical cancer patients at an early stage, patients with recurrent/metastatic cervical cancer have a poor prognosis, which is an important cause of death. In patients with lymph node metastasis and/or disease progression at local lesion, the recurrence rate is as high as 28-64% ( 9, 10). Among the cervical cancer patients at early stage without evidence of lymph node metastasis, approximately 11-22% of them relapse after receiving primary standard treatment. The treatment of cervical cancer is mainly determined by the disease stage at diagnosis, and the commonly used methods include surgical resection, radiotherapy and chemotherapy ( 8). According to the statistics by World Health Organization, in 2020, an estimated 604,127 women will be diagnosed with cervical cancer globally and 341,831 women will die from the disease, with approximately 90% of the cases occurring in low- and middle-income countries ( 7). With the popularization of HPV vaccine and applying systemic screening of disease in recent years, the incidence of cervical cancer has decreased significantly in developed countries ( 2– 4), but the incidence and mortality rate in developing countries still remained high ( 5, 6). It is the fourth most common cancer in women worldwide and the fourth leading cause of cancer-related death ( 1). Systematic Review Registration:, identifier CRD42021291723.Ĭervical cancer is the most common malignancy in female reproductive system. The results from recent high-quality clinical trials are expected to validate these findings. The total incidence of the adverse events of grade 3 and above was 0.212 (95% CI: 0.065-0.509).Ĭonclusions: Pembrolizumab provides significant benefits in response rate and survival for cervical cancer patients. Main adverse events included abnormal liver function, hypothyroidism, neutropenia, anemia, decreased appetite, fatigue, fever, etc. Results: A total of 7 studies with 727 patients were included.
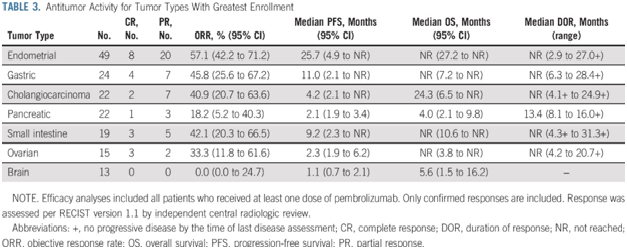
Outcomes included complete response (CR), partial response (PR), stable disease (SD), disease progression (PD), objective response rate (ORR), disease control rate (DCR), overall survival (OS), progression-free survival (PFS), the best time to response (TTR), death rate, adverse events (AE). Methods: PubMed, Embase, Cochrane Library and Web of Science were searched for literatures published until October 31, 2021. Therefore, we performed this meta-analysis on existing studies to reveal the efficacy and safety of pembrolizumab in treating cervical cancer. Multiple clinical trials have been conducted, and some of them have shown good results as expected. Some high-quality clinical trials have studied the effect of applying pembrolizumab in treating cervical cancer. Department of Gynecology and Obstetrics, Yantai Yuhuangding Hospital Affiliated to Qingdao University, Yantai, Chinaīackground: According to current research, the objective response rate and overall survival of pembrolizumab in the treatment of several types of solid tumors have been significantly improved.Lin Qi *, Ning Li, Aimin Lin, Xiuli Wang and Jianglin Cong *


 0 kommentar(er)
0 kommentar(er)
